Author Guidelines
AUTHOR GUIDELINES
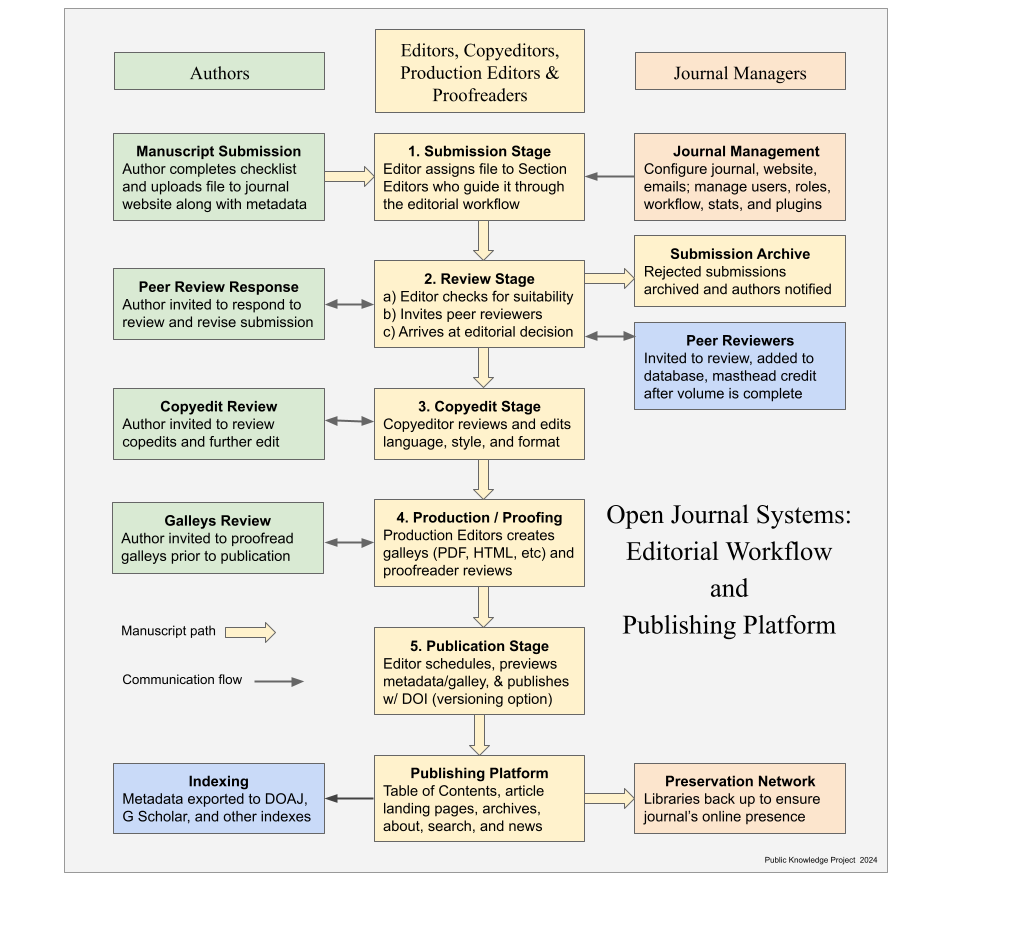
Authors are invited to make a submission to this journal. All submissions will be assessed by an editor to determine whether they meet the aims and scope of this journal. Those considered to be a good fit will be sent for peer review before determining whether they will be accepted or rejected.
Before making a submission, authors are responsible for obtaining permission to publish any material included with the submission, such as photos, documents and datasets. All authors identified on the submission must consent to be identified as an author. Where appropriate, research should be approved by an appropriate ethics committee in accordance with the legal requirements of the study's country.
An editor may desk reject a submission if it does not meet minimum standards of quality. Before submitting, please ensure that the study design and research argument are structured and articulated properly. The title should be concise and the abstract should be able to stand on its own. This will increase the likelihood of reviewers agreeing to review the paper. When you're satisfied that your submission meets this standard, please follow the checklist below to prepare your submission.
 Submission Preparation Checklist
Submission Preparation Checklist
All submissions must meet the following requirements.
- This submission meets the requirements outlined in the Author Guidelines.
- This submission has not been previously published, nor is it before another journal for consideration.
- All references have been checked for accuracy and completeness.
- All tables and figures have been numbered and labeled.
- Permission has been obtained to publish all photos, datasets and other material provided with this submission.
Section default policy
Privacy Statement
The names and email addresses entered in this journal site will be used exclusively for the stated purposes of this journal and will not be made available for any other purpose or to any other party.
- Submission
- Aims and Scope
- Manuscript Categories and Requirements
- Data Protection
- Preparing the Submission
- Peer Review, Editorial Policies and Publication Ethics
- Author Licensing and Open Access fees
- Publication Process after Acceptance
- Post Publication
- Editorial Office Contact Details
1. SUBMISSION
New submissions should be made via the Research Exchange submission portal. You may check the status of your submission at any time by logging on to https://healthpromotionjournal.ir/index.php/hps/about/submissions and clicking the “My Submissions” button. For technical help with the submission system, please review our FAQs or contact HPS@thums.ac.ir.
Format submission:
Manuscript format template in Word
Health promotion science now offers Free Format submission for a simplified and streamlined submission process. Before you submit, you will need:
- Your manuscript: this should be an editable file including text, figures, and tables, or separate files – whichever you prefer. All required sections should be contained in your manuscript, including abstract (which does need to be correctly styled), introduction, methods, results, and conclusions. Figures and tables should have legends. Figures should be uploaded in the highest resolution possible. If the figures are not of sufficiently high quality your manuscript may be delayed. References may be submitted in any style or format, as long as it is consistent throughout the manuscript. Supporting information should be submitted in separate files. If the manuscript, figures or tables are difficult for you to read, they will also be difficult for the editors and reviewers, and the editorial office will send it back to you for revision. Your manuscript may also be sent back to you for revision if the quality of English language is poor.
- An ORCID ID, freely available at https://orcid.org (Why is this important? Your article, if accepted and published, will be attached to your ORCID profile. Institutions and funders are increasingly requiring authors to have ORCID IDs.)
- The title page of the manuscript, including:
- Your co-author details, including affiliation and email address. (Why is this important? We need to keep all co-authors informed of the outcome of the peer review process.)
- Statements relating to our ethics and integrity policies, which may include any of the following (Why are these important? We need to uphold rigorous ethical standards for the research we consider for publication):
- data availability statement
- funding statement
- conflict of interest disclosure
- ethics approval statement
- patient consent statement
- permission to reproduce material from other sources
- clinical trial registration
To submit, login at https://healthpromotionjournal.ir/index.php/hps/about/submissions and create a new submission. Follow the submission steps as required and submit the manuscript.
Authors should kindly note that submission implies that the content has not been published or submitted for publication elsewhere except as a brief abstract in the proceedings of a scientific meeting or symposium. Health promotion science will consider submissions that have been made available online previously, either on a preprint server or on the authors’ own website.
- Conflict of Interest Statement
Upon submission, authors will be asked to affirm a conflict of interest statement. For guidance, see the ‘Conflict of Interest’ section in the Editorial Policies and Ethical Considerations section below. Authors should ensure they liaise with all co-authors to confirm agreement with the statement.
- For help with submissions, please contact: info@healthpromotionjournal.ir
N.B. Health promotion science employs a plagiarism detection system. By submitting your manuscript, you accept that it will be screened for plagiarism against previously published works.
Refer and Transfer Program
Health promotion science believes that no valuable research should go unshared. This journal participates in PKP’s Refer & Transfer program. If your manuscript is not accepted, you may receive a recommendation to transfer your manuscript to another suitable PKP journal, either through a referral from the journal’s editor or through our Transfer Desk Assistant.
- AIMS AND SCOPE
Philosophy
Health Promotion Science is founded on the principle that all rigorously conducted, data-driven research in health promotion and related fields holds intrinsic value. We are committed to advancing global health by disseminating evidence-based knowledge, fostering interdisciplinary collaboration, and promoting equitable access to scientific discoveries.
Scope
Health Promotion Science is an international, open-access, peer-reviewed journal dedicated to publishing high-quality research across the spectrum of health promotion, health education, public health, and allied disciplines. The journal prioritizes scientific integrity, innovation, and practical relevance, welcoming contributions that address pressing health challenges and advance theoretical, clinical, translational, and policy-oriented knowledge.
Key Research Themes
The journal covers, but is not limited to, the following areas:
- Health Education: Behavioral interventions, health literacy, school- and community-based education programs, and digital health communication.
- Public Health: Epidemiology, disease prevention, vaccination strategies, and health system strengthening.
- Mental and Behavioral Health: Psychosocial interventions, stigma reduction, mental health promotion, and addiction prevention.
- Environmental Health: Climate change impacts, pollution control, sustainable practices, and One Health approaches.
- Health Policy and Advocacy: Policy analysis, health governance, legislative impacts, and community-driven advocacy initiatives.
- Health Equity: Social determinants of health, disparities in marginalized populations, culturally sensitive interventions, and inclusive healthcare models.
- Innovative Technologies: Digital health tools, AI applications, telemedicine, and wearable devices for health monitoring.
- Global and Planetary Health: Cross-border health initiatives, humanitarian interventions, and sustainable development goals (SDGs).
- Clinical and Palliative Care: Patient-centered care models, survivorship programs, and quality-of-life improvements.
- Interdisciplinary Research: Integration of healthcare, education, policy, and social sciences to address complex health challenges.
Inclusive Research Criteria
We actively encourage submissions that reflect diverse methodologies and perspectives, including:
- Pilot Studies: Scientifically robust preliminary investigations evaluating feasibility, innovative theories, or novel interventions.
- Incremental Advances: Studies expanding current knowledge, challenging paradigms, or offering new directions.
- Negative/Null Findings: Rigorously conducted research with non-significant outcomes to counter publication bias.
- Replication Studies: Confirmatory or contradictory studies reinforcing reproducibility in health promotion research.
- Emerging Relevance: Exploratory work with indirect or prospective implications for fields such as cancer prevention, chronic disease management, or global health security.
Article Types
- Original Research: Full-length empirical studies.
- Brief Reports: Concise findings with immediate relevance.
- Methodological Advances: Novel research tools, models, or frameworks.
- Clinical Study Reports: Outcomes of intervention trials or observational studies.
- Registered Reports: Peer-reviewed protocols for hypothesis-driven research.
- Case Studies: Context-specific insights with broader applicability.
- Reviews: Systematic, scoping, or narrative reviews synthesizing evidence.
- Commentaries & Editorials: Expert perspectives commissioned by the Editor-in-Chief.
Evaluation Criteria
Submissions are assessed based on:
- Scientific Rigor: Methodological transparency, statistical validity, and adherence to ethical standards (e.g., IRB approval, informed consent).
- Evidence-Based Conclusions: Clear alignment between data and interpretations.
- Impact: Potential to inform practice, policy, or future research.
For inquiries regarding topics not listed above, contact: info@healthpromotionjournal.ir
Health Promotion Science serves as a catalyst for transformative change, bridging the gap between research and real-world health outcomes. We invite scholars, practitioners, and policymakers to contribute to a collaborative, evidence-driven future in global health.
- MANUSCRIPT CATEGORIES AND REQUIREMENTS
Health Promotion Science welcomes submissions across diverse formats to advance the field through rigorous, impactful, and ethically sound research. Below are the manuscript categories, requirements, and guidelines for authors:
Accepted Manuscript Types
- Original Research Article
- Scope: Comprehensive studies contributing novel insights to health promotion, health education, public health, or allied fields. Includes:
- Pilot, replication, or incremental studies.
- Negative/null findings with methodological rigor.
- Meta-analyses or interdisciplinary work bridging theory and practice.
- Structure: Abstract (250 words), Introduction, Methods, Results, Discussion, Conclusion, References.
- Requirements: Clear hypotheses, robust methodology (e.g., IRB approval for human studies), and data accessibility statements.
- Scope: Comprehensive studies contributing novel insights to health promotion, health education, public health, or allied fields. Includes:
- Brief Report
- Scope: Concise, high-impact studies with preliminary or focused findings (e.g., innovative interventions, policy evaluations, or emerging trends).
- Structure: Same as Original Articles but condensed (recommended 1,500–3,000 words).
- Requirements: ≤3 tables/figures; emphasize practical applications or paradigm-shifting implications.
- Clinical Study Report
- Scope: Retrospective/prospective clinical trials, observational studies, or intervention analyses in health promotion contexts (e.g., behavioral therapies, community health programs).
- Structure: Abstract, Introduction, Methods (including trial registration number, e.g., ClinicalTrials.gov), Results, Discussion, Ethics Statement.
- Requirements: Adherence to CONSORT, STROBE, or other relevant guidelines.
- Registered Report
- Scope: Hypothesis-driven studies with pre-registered protocols (Stage 1: peer-reviewed proposal; Stage 2: post-data-collection final submission).
- Requirements: In-principle acceptance granted before data collection. Learn more.
- Case Report
- Scope: Detailed analyses of unique patient/community cases with broader lessons for health promotion (e.g., cultural competency challenges, rare health disparities).
- Structure: Abstract, Introduction, Case Description, Discussion, Ethical Considerations.
- Requirements: Patient consent, anonymized data, and alignment with CARE guidelines.
- Method Report
- Scope: Novel methodologies, tools, or improvements to existing techniques (e.g., AI-driven health assessments, community engagement frameworks).
- Structure: Abstract, Introduction, Method Description, Validation, Applications, Limitations.
- Requirements: Comparative analysis with conventional methods; open-access protocols/tools encouraged.
- Review Article
- Scope: Systematic, scoping, or narrative reviews synthesizing evidence on emerging topics (e.g., digital health equity, climate change impacts on mental health).
- Structure: Structured abstract, Introduction, Thematic Synthesis, Gaps/Future Directions, Conclusion.
- Requirements: PRISMA or PRISMA-ScR compliance for systematic/scoping reviews; explicit search strategy.
- Editorials, Commentaries, and Perspectives
- Scope: Commissioned by the Editor-in-Chief to address urgent issues, policy debates, or interdisciplinary collaborations.
- Length: 800–1,500 words.
General Guidelines
- Ethical Standards: All studies involving human participants must include IRB approval, informed consent, and conflict-of-interest declarations.
- Data Transparency: Raw data/code must be shared via public repositories (e.g., OSF, Dryad) unless legally restricted.
- Accessibility: Submissions in clear, concise English; language editing certificates required for non-native speakers.
- Formatting: No strict word limits, but brevity is prioritized. Use Endnote File Journal for references.
Criticism and Rebuttals
- Commentaries on Published Work: Concise critiques (≤1,000 words) addressing significant errors or reinterpretations of prior research.
- Process: Submit via editorial office; undergoes peer review. Published only if evidence substantiates major claims require correction.
- Author Response: Original authors may submit a rebuttal (≤500 words) alongside the critique.
Publication Fees and Licensing
Article Processing Charge (APC)
There are no fees for submitting an article or for the peer review process. To cover the costs of open access publication, an Article Processing Charge (APC) is applied to all manuscripts after they have been accepted for publication.
The APC is 50 EUR for international authors and 3,000,000 IRR for Iranian authors. Waivers are available for authors from low-income regions. For a complete breakdown of our fees and policies, please view our detailed Publishing Fees Statement.
Publishing Fees Statement
(Last Updated: 2025-03-18)
Health promotion science has no Fees or Charges for submission and regular peer review.
All accepted articles must pay the publishing fee after acceptance:
50 Euro per article (for non-Iranian authors)
3,000,000 IRR per article (for Iranian authors for the 2025 Issues)
- Account Holder Name: Mohammad Hossein Delshad
- Bank Name: Parsian Bank
- Account Number: 80001323086002
- Sheba (IBAN): IR700540104680001323086002
- Card Number (for card-to-card transfer): 6221-0610-6760-4423
After completing the transfer, please send a scanned copy or screenshot of the payment receipt, along with your manuscript ID, to our email address: info@healthpromotionjournal.ir. This will help us confirm your payment promptly.
To learn about our waiving policies, please contact the journal office.
Note: For an article to be considered "Iranian," the following three criteria must be met:
The first author's affiliation must be in Iran.
The corresponding author's affiliation must be in Iran.
At least half of all authors' affiliations must be in Iran.
- Licensing: All articles published under CC BY-NC 4.0unless otherwise requested. Copyright Policy.
- DATA PROTECTION AND PRIVACY
Health Promotion Science and its publisher are committed to safeguarding the privacy and security of all personal data collected during the manuscript submission, peer review, and publication processes. We adhere to global data protection regulations, including the General Data Protection Regulation (GDPR), and uphold ethical standards for data transparency and user rights.
Data Collection and Use
Purpose: Personal information (e.g., names, email addresses, institutional affiliations, ORCID IDs) is collected to facilitate:
Manuscript submission, peer review, and editorial workflows.
Communication with authors, reviewers, and editors.
Dissemination of published work through indexing services, databases, and partner platforms.
Lawful Basis: Data processing is conducted under contractual necessity (e.g., publishing agreements) and legitimate interests (e.g., maintaining academic integrity).
Data Sharing
Third Parties: Data may be shared with trusted partners, including:
Publishing Platforms (e.g., PKP/OJS for hosting and archiving).
Indexing Services (e.g., PubMed, Scopus) to enhance article visibility.
Peer Reviewers: Reviewer identities remain confidential unless open peer review is explicitly opted for.
Anonymity: Author and reviewer identities are protected during double-blind peer review.
Data Security
Measures: We employ industry-standard protocols to ensure data security, including:
Encryption of sensitive information during transmission and storage.
Restricted access to personal data (limited to editorial staff and essential service providers).
Regular security audits of our publishing infrastructure.
Your Rights
You retain the right to:
Access: Request a copy of your personal data held by the journal.
Correction: Update inaccuracies in your profile or submissions.
Deletion: Request removal of personal data, subject to legal and archival obligations.
Objection: Opt out of non-essential communications (e.g., promotional emails).
To exercise these rights, contact info@healthpromotionjournal.ir.
Data Retention
Personal data is retained only as long as necessary for operational, legal, or archival purposes. Published articles remain permanently accessible as part of the scholarly record.
Special Considerations
Minors/Vulnerable Groups: Submissions involving minors require explicit consent from guardians, in alignment with ethical guidelines.
Cross-Border Transfers: Data may be processed globally but is protected under GDPR-compliant agreements with service providers.
Updates to This Policy
Changes to our data protection practices will be communicated via the journal website. For details on PKP’s privacy standards, visit PKP’s Privacy Policy.
By engaging with Health Promotion Science, you acknowledge and consent to the responsible use of your data as outlined above. For questions, contact our Data Protection Officer at info@healthpromotionjournal.ir.
- PREPARING YOUR SUBMISSION
Health Promotion Science prioritizes clarity, reproducibility, and ethical rigor. Below are guidelines to ensure your submission aligns with journal standards:
Initial Submission Formats
- Accepted Formats:
- Microsoft Word: Text, tables, and figures embedded ORas separate files.
- PDF: Single PDF containing text, tables, and figures.
- Post-Acceptance Requirements:
- Editable Word file with text.
- Figures as separate high-resolution files (TIFF/PNG/JPEG).
- Tables in editable Word or Excel format.
Note for Transfers/Resubmissions:
- Articles transferred from other journals or resubmitted after prior review do notrequire reformatting.
Revisions/Resubmissions
Response to Reviewers:
- Submit a Rebuttal Letteras a separate document.
- Format: Copy reviewer/editor comments into a Word file and insert responses below each point using >>>.
- Revised Manuscript:
- Upload a version with tracked changes(for “major revision” decisions).
- Figures:
- Provide updated figures as separate files.
General Guidelines
- Language:
- Use US English(converted during production if submitted in British English).
- Footnotes:
- Not permitted; integrate essential notes parenthetically into the text.
- Cover Letter:
- Optional but recommended for context (e.g., novelty, ethical considerations).
Manuscript Structure
Main Text File
Organize in the following order:
- Title Page:
- Title: Concise, keyword-rich (avoid abbreviations).
- Authors: Full names, affiliations, ORCIDs. Indicate current addressif different.
- Corresponding Author: Email, phone, and postal address.
- Abstract & Keywords:
- Abstract(≤300 words): Structured by article type:
- Original Research/Clinical Study/Method Report:
Background, Aims, Methods & Results, Conclusion(add clinical trial registration numberfor trials). - Review:
Background, Recent Findings, Conclusion. - Case Report:
Background, Case, Conclusion.
- Original Research/Clinical Study/Method Report:
- Keywords: 2–6 terms (MeSH terms preferred).
- Abstract(≤300 words): Structured by article type:
- Main Text:
- Original Research/Clinical Study/Method Report:
Introduction, Methods, Results, Discussion. - Review:
Introduction, Thematic Subheadings, Conclusion. - Case Report:
Introduction, Case Description, Discussion.
- Original Research/Clinical Study/Method Report:
- Ethical Statement(Mandatory from February 1, 2025):
- Include:
- IRB/ethics committee approval details.
- Consent for participation and publication (e.g., patient consent for case reports).
- Pre-2025 Submissions: Ethical details in Methods section.
- Include:
- Data Availability Statement:
- Required for all accepted manuscripts:
- Share data via repositories (e.g., Dryad, Figshare) with a persistent identifier(DOI/accession number).
- Use FAIR principles(as open as possible, as closed as necessary).
- Sample statements: [LINK].
- Required for all accepted manuscripts:
- Acknowledgments:
- Recognize non-author contributors (with permission) and funders.
- Exclude: Anonymous reviewer acknowledgments.
Additional Policies
- Authorship: Follow ICMJE criteria (see Editorial Policies).
- Conflict of Interest: Declare all financial/non-financial interests.
- February 1, 2020: All submissions must include a standalone Ethical Statement(approvals, consent).
- Pre-2020 submissions: Include ethics details in the Methods Manuscripts lacking ethical statements, consent confirmations, or trial registration details will be returned or rejected.
- PEER REVIEW, EDITORIAL POLICIES AND PUBLICATION ETHICS
- Manuscript Evaluation Criteria and Peer Review
Preprints: Permitted but disclose upon submission.
- Conflicts of Interest (CoI)
All authors must disclose any financial, personal, or professional relationships that could be perceived as influencing the research. This includes, but is not limited to:
- Funding sources (grants, sponsorships).
- Employment or advisory roles.
- Patents, stock ownership, or royalties.
- Personal relationships or competing academic interests.
Submission Requirements:
- Include a statement titled “Conflict of Interest”in the manuscript, even if no conflicts exist (e.g., “The authors declare no conflicts of interest”).
- Ensure all co-authors review and approve the final statement.
- For detailed guidance, refer to the Editorial Policies and Ethical Considerations.
- Authors’ Contributions
specify individual contributions using the CRediT Taxonomy. Examples include:
- Conceptualization, Methodology, Formal Analysis.
- Investigation, Data Curation, Writing – Original Draft.
- Visualization, Supervision, Funding Acquisition.
Format:
plaintext
Author A: Conceptualization, Writing – Review & Editing.
Author B: Methodology, Formal Analysis.
Author C: Data Curation, Visualization.
References
Style: Endnote File Journal (numbered superscript in-text, e.g., Data were analyzed¹).
Key Elements:
- Journal Articles: Author(s). Article title. Journal Abbreviation. Year;Volume(Issue):Page. DOI.
- Example: Smith A, Jones B. Health equity in urban settings. *Health Educ Res*. 2023;12(3):45-56. doi:10.1234/her.2023.1234.
- Books: Author(s). Book Title. Edition. Publisher; Year.
- Online Resources: Include DOI if available; otherwise, add access date (e.g., Accessed March 15, 2024).
Notes:
- Use MEDLINE/Index Medicus abbreviations for journals.
- Avoid formatting (italics/bold) in submissions; focus on completeness.
- Tables
Requirements:
- Submit as editable Word/Excel files(no images).
- Titles and footnotes must enable standalone understanding.
- Define all abbreviations and statistical terms (e.g., SD, SEM) in footnotes.
- Footnote symbols: Use †, ‡, §, ¶ (in order); reserve *, **, *** for p-values.
Example:
Table 1. Baseline Characteristics of Study Participants
Characteristic | Group A (n=50) | Group B (n=50)
---------------|---------------|---------------
Age, mean ± SD | 45.2 ± 12.1† | 47.8 ± 10.3
BMI, kg/m² | 26.4 ± 3.2 | 25.9 ± 4.1‡
†SD = standard deviation; ‡BMI = body mass index.
Figure Legends
Structure:
- Title: Briefly describe the figure.
- Description: Explain methods, key findings, and annotations.
- Definitions: Clarify symbols, abbreviations, and units.
Example:
**Figure 1. Impact of Community Interventions on Smoking Cessation**
Kaplan-Meier curves showing smoking cessation rates over 12 months. ▲: Control group; ■: Intervention group. Error bars represent SEM. SEM = standard error of the mean.
Additional Notes:
- Reproducibility: Align with the journal’s FAIR data policy (see Data Availability Statement).
- Ethical Alignment: Ensure consistency with ethical approvals (section iv).
For further details, consult the Endnote File Journal and Reference Style Guide.
Authors’ Contributions
Specify individual contributions using the CRediT Taxonomy. Examples include:
Minimum Reference Requirements
Ensure references are complete and formatted according to Endnote File Journal style. Key elements for different source types:
|
Journal Article |
Book |
Book Chapter |
|
- Full author names |
- Full author names |
- Full author names |
|
- Year of publication |
- Year of publication |
- Year of publication |
|
- Article title |
- Book title |
- Chapter title |
|
- Journal title (preferably unabbreviated) |
- Place of publication |
- Editor(s)/Book author |
|
- Volume/Issue |
- Publisher |
- Book title |
|
- Page range |
- Page numbers (if citing specific sections) |
- Place of publication |
|
- Include DOI if available |
|
- Publisher |
|
|
|
- Page range |
Example:
- Smith AB, Jones CD. Community-based interventions for diabetes prevention. *Health Education Research*. 2023;38(5):120-135. doi:10.1234/her.2023.5678.
Figures
Initial Submission:
- Acceptable formats: JPEG, PNG, TIFF, PDF (embedded in manuscript or separate files).
- Resolution: ≥150 dpi for peer review.
Post-Acceptance:
- Line Art/Graphs: Black-and-white preferred (ensure legibility in grayscale).
- Color Figures: Free for online publication; avoid color-dependent interpretations.
- High Resolution: ≥300 dpi for TIFF/PDF; vector formats (e.g., EPS) for line art.
- Legends: Include in main text file; define symbols/abbreviations.
Additional Files
- Appendices:
- Submit as separate files (e.g., "Appendix A.docx").
- Reference in-text (e.g., See Appendix 1 for survey details).
- Supporting Information:
- Non-essential data (e.g., extended datasets, videos, code).
- Hosted online without editing; cite in-text (e.g., Table S1).
- Public Data Repositories: Include accession numbers/DOIs (e.g., Data available via Dryad: doi:10.5061/dryad.xxxx).
General Style Guidelines
- Abbreviations:
- Define at first mention (Body Mass Index [BMI]) and use abbreviation thereafter.
- Units:
- Use SI units(e.g., kg, mL, mmol/L). Consult BIPM.
- Chemical/Drug Names:
- Generic names only (e.g., paracetamol, not Tylenol®). Mention manufacturers in parentheses if proprietary products are used.
Resource Identification Initiative (RII)
Health Promotion Science endorses the Research Resource Identifiers (RRIDs) to enhance reproducibility.
How to Cite Resources:
- Antibodies:
- Anti-p53 antibody (Vendor: ABC Bioscience, Cat# AB123, RRID:AB_123456).
- Software/Databases:
- R Statistical Software (https://www.r-project.org/, RRID:SCR_001905).
- Model Organisms:
- Mus musculus (RRID:IMSR_JAX:000664).
Steps to Obtain RRIDs:
- Visit the Resource Identification Portal.
- Search for the resource (antibody, software, organism).
- Click "Cite This" to generate the RRID citation.
- Register Unlisted Resources: Contact rii-help@scicrunch.org.
Include RRIDs in:
- First mention in Methods.
- Keywordssection (e.g., Keywords: health equity, RRID:AB_123456).
Ethical & Compliance Notes
- Data Availability: Adhere to FAIR principles (Findable, Accessible, Interoperable, Reusable).
- Ethical Approvals: Mandatory for human/animal studies (see Section 5.iv).
Manuscript Preparation Tips
To enhance the quality, visibility, and impact of your submission, Health Promotion Science recommends the following:
Writing for SEO:
Optimize your title, abstract, and keywords with high-impact terms relevant to health promotion (e.g., "health equity," "behavioral interventions," "community health").
Use Best Practices for SEO to improve discoverability.
Structure Clarity:
Ensure logical flow between sections (e.g., methods → results → discussion).
Use subheadings to improve readability, especially in review articles.
Reproducibility:
Follow FAIR data principles for shared datasets.
Include detailed protocols for methods or interventions.
Editing and Formatting Support
Health Promotion Science collaborates with Editing Services to help authors refine their submissions:
Language Editing:
Professional English language polishing for grammar, clarity, and academic tone.
Certification provided for submissions requiring language validation.
Translation Services:
Manuscript translation into English by subject-area experts.
Formatting Assistance:
Compliance with journal-specific guidelines (e.g., reference style, figure formatting).
Artwork Preparation:
High-resolution figure creation/optimization for print and digital formats.
Benefits:
Increased readability and adherence to technical standards.
Higher likelihood of acceptance through improved presentation.
Access Editing Services
Ethical and Technical Compliance
Pre-Submission Checklist:
Confirm inclusion of ethical statements, data availability, and conflict of interest declarations.
Validate reference formatting ( Endnote File Journal style) and figure/table accessibility.
ORCID Integration:
Register and link ORCID iDs for all authors to ensure proper attribution.
- The acceptance criteria for all papers are scientific rigor, evidence that the data support conclusions, adherence to technical and ethical standards and the scope of the journal. Manuscripts are single-blind peer reviewed in which peer reviewer identities are kept confidential. Health promotion science's policy on confidentiality of the review process is available here. The manuscript evaluation criteria and peer review process is applicable to all manuscripts submitted to health promotion science, irrespective of their mode of submission which may be direct, invited (e.g. for special issues) or transferred from another Health promotion science supporter journal. For more detailed version of our evaluation criteria and peer review policy, please click here.
- Manuscripts submitted by Board Members
- We welcome manuscripts submitted by our Associate Editors and Advisory Board members. These, however, are not given priority over other manuscripts, and Board Member status has no bearing on editorial consideration. All submissions are handled, in their entirety, by the Editor-in-Chief and the Board Member(s) will have no involvement in the editorial process of manuscripts submitted to the journal, no access to any confidential information, and have no input into the final decisions made by the Editor-in-Chief.
- Author Suggested Reviewers
- Authors are required to suggest at least three names of recommended peer reviewers and their email addresses. Authors can also indicate reviewers to exclude, with associated reasons. The editors reserve the right to accept or decline these requests.
- Peer-review Reports from Manuscript Transfers
- Health promotion science will consider the peer-review reports (if available) from its partner journals; however, the Editor-in-Chief may choose to send such manuscripts for additional external review. Criteria for accepting these manuscripts will be same as for the direct submission to health promotion science.
- Information for Peer-reviewers
- We are extremely grateful to all our peer reviewers for their help in publication of excellent Health promotion research. Please note that Health promotion science will accept papers solely on the bases of scientific rigor, adherence to technical and ethical standards, and evidence that the data support the conclusions, with no requirement for perceived impact.
- In accordance with the guidelines issued by the World Association of Medical Editors “Reviews will be expected to be professional, honest, courteous, prompt, and constructive.” Click here to learn more about reviewing a journal article.
- Recognition for Reviewers: A reviewer’s input to the editorial process is invaluable. To help recognize the importance of reviewing, Wiley has partnered with Publons to give you official recognition for your peer review work. This partnership means you can opt-in to have your reviews for Health promotion science automatically added to your reviewer profile on Publons. Click here for additional details. As a sign of gratitude for their activity, Health promotion science will reward all its active reviewers with a designer certificate and host their names in the year’s last issue.
- EDITORIAL POLICIES
- Preprints
- Health Promotion Scienceencourages transparency and rapid dissemination of research. We welcome submissions previously shared on preprint servers(e.g., medRxiv, bioRxiv, arXiv, SSRN) or personal/ institutional websites, provided they have not been formally published (e.g., in a journal, book, or conference proceedings).
- Requirements for Preprint Submissions:
- Disclosure: Authors must notify the editorial office at submission if the manuscript is available as a preprint.
- Post-Acceptance Actions:
- Linking Preprints: Update the preprint record with a direct link to the published article’s DOI (e.g., “This work is now published in [Journal Name] [DOI link]”).
- Citation Policy: Cite only the final published version in subsequent work to consolidate academic impact.
- Open Access Compliance: Accepted preprints must adhere to the journal’s PKP Open Access Agreement and associated fees.
ORCID Integration
To enhance authorship transparency and combat name ambiguity, Health Promotion Science requires:
- Submitting Author: Must provide a valid ORCID iDduring manuscript submission.
- All Co-Authors: Strongly encouraged to link ORCIDs to their affiliations and contributions.
Benefits of ORCID:
- Ensures accurate attribution of scholarly work.
- Streamlines manuscript processing and indexing.
- Complies with funder/institutional mandates.
Register or Connect Your ORCID iD (Takes 2 minutes).
Ethical Alignment
These policies uphold the journal’s commitment to:
- Integrity: Preventing dual publication and citation fragmentation.
- Transparency: Linking preprints to final versions for traceability.
- Accountability: Crediting researchers unambiguously via ORCID.
Non-Compliance: Submissions failing to meet these requirements will be returned for correction or rejected.
- DATA SHARING & ACCESSIBILITY
Health Promotion Sciencechampions open science and reproducibility. Authors must adhere to the following policies to ensure research transparency and utility:
Data Sharing Policy
- Mandatory Archiving:
- All data underlying published results must be deposited in a public repositorythat guarantees preservation (e.g., Dryad, Figshare, Zenodo).
- Use the re3data registry to select an appropriate repository.
- FAIR Compliance:
- Data must be Findable, Accessible, Interoperable, and Reusable(FAIR principles).
- Even if access is restricted (e.g., for privacy/legal reasons), provide metadata with a clear justification.
- Data Availability Statement:
- Include this statement in the manuscript header. Examples:
- “Data are available in [Repository Name] under DOI [link].”
- “Data cannot be shared due to [ethical/legal restrictions]. Access inquiries may be directed to [contact].”
- View detailed examples of acceptable statements.
- Include this statement in the manuscript header. Examples:
Cell Line Authentication
To uphold research integrity, Health Promotion Science requires:
- Mandatory Disclosure in Methods:
- Source: Where cell lines were obtained (e.g., ATCC, DSMZ).
- Authentication: Whether testing was performed, including:
- Method(e.g., STR profiling, isoenzyme analysis).
- Dateof last authentication.
- Exceptions:
- No re-authentication needed if:
- Cells were obtained from a certified cell bank(e.g., ATCC).
- Used within 6 monthsof receipt/resuscitation.
- Include the cell bank’s characterization method (e.g., “ATCC performed STR profiling”).
- No re-authentication needed if:
- Best Practices:
- Cross-check DNA profiles with donor tissue or established cell lines.
- Use ICLACresources for combating misidentified cell lines.
Non-Compliance:
- Submissions lacking data availability statements or cell line authentication details will be returned for revision.
- Persistent non-compliance may result in rejection or post-publication corrections.
This policy ensures alignment with global standards for transparency in health promotion research. For queries, contact info@healthpromotionjournal.ir.
- ETHICAL APPROVALS AND CONSENT TO PARTICIPATE
Health Promotion Scienceupholds the highest ethical standards in research involving humans, animals, and clinical trials. Authors must adhere to the following guidelines:
Human Studies
- Ethics Approval:
- Include a statement in the Methodssection confirming:
- Name of the ethics committee/institutional review board (IRB) that approved the study.
- Compliance with recognized standards (e.g., Declaration of Helsinki, ICH Good Clinical Practice).
- Example: “This study was approved by the [Institution] Ethics Committee (Protocol #XYZ) and adhered to the Declaration of Helsinki.”
- Include a statement in the Methodssection confirming:
- Informed Consent:
- Participant Data/Images: Explicit consent must be obtained for identifiable information (e.g., photographs, case details).
- Confirmation: Authors must attest to consent during submission; no need to submit forms.
- Template: Use Patient Consent Formif required.
Animal Studies
- Ethics Statement:
- Declare in the Methodssection:
- Approval body (e.g., institutional animal care committee).
- Adherence to national/institutional guidelines (e.g., NIH Guide for Care and Use of Laboratory Animals).
- Example: “Procedures were approved by the [Institution] Animal Ethics Committee (Protocol #ABC) and complied with Directive 2010/63/EU.”
- Declare in the Methodssection:
- Reporting Standards:
- Follow the ARRIVE Guidelinesfor rigor and transparency.
- Specify housing, husbandry, and humane endpoints.
Clinical Trial Registration
- Mandatory Registration:
- Prospective registration in public databases (e.g., ClinicalTrials.gov, ISRCTN, WHO ICTRP).
- Include the trial registry name and numberat the end of the abstract.
- Example: “ClinicalTrials.gov Identifier: NCT12345678.”
- Retrospective Registration:
- Explain reasons for delayed registration (e.g., oversight, legal constraints).
Randomized Controlled Trials (RCTs)
- Reporting Requirements:
- State the randomization method(e.g., block randomization, computer-generated sequence).
- Label as an RCT in the title and abstract(e.g., “A Randomized Controlled Trial of…”).
- Compliance:
- Follow the CONSORT Checklistand include a flow diagram.
Key Dates
- February 1, 2020: All submissions must include a standalone Ethical Statement(approvals, consent).
- Pre-2020 submissions: Include ethics details in the Methods
Non-Compliance:
- Manuscripts lacking ethical statements, consent confirmations, or trial registration details will be returned or rejected.
This policy ensures alignment with global ethical norms in health promotion research. For guidance, contact info@healthpromotionjournal.ir.
- RESEARCH REPORTING GUIDELINES
Health Promotion Science mandates adherence to globally recognized reporting standards to ensure methodological transparency, reproducibility, and ethical rigor.
Key Reporting Guidelines
Human Studies:
CONSORT: For randomized controlled trials (include flow diagrams).
STROBE: For observational studies (cohort, case-control, cross-sectional).
SPIRIT: For clinical trial protocols.
CARE: For case reports.
COREQ: For qualitative research.
Systematic Reviews/Meta-Analyses: PRISMA (include flowcharts and search strategies).
Animal Studies: ARRIVE 2.0 (detailed methods, ethical oversight, welfare).
Diagnostic/Prognostic Studies: STARD or TRIPOD.
Economic Evaluations: CHEERS.
General Standards: Consult the EQUATOR Network or FORCE11 for discipline-specific guidance.
Species Nomenclature
First Mention: Use the common name followed by the scientific name (genus species) in parentheses (e.g., Norway rat (Rattus norvegicus)).
Exceptions: Omit scientific names for well-known species (e.g., Homo sapiens) in titles.
Tumor Classification and Staging
AJCC/UICC TNM System:
Specify stage groupings (e.g., Stage IIA [T2 N0 M0]).
Provide verbal translations of T/N/M classifications (e.g., T2: tumor >2 cm but ≤5 cm).
Cite the staging manual used (e.g., AJCC Cancer Staging Manual, 8th Edition).
Non-Standard Staging: Include TNM equivalents.
Display: Present sequences as aligned figures, not tables.
Chemical Structures
Disclosure:
Provide exact structures and synthesis protocols for novel compounds.
Include experimental details (e.g., reagents, reaction conditions) in the Methods.
References: Cite patents or prior publications describing synthesis.
Data Analysis Best Practices
Statistical Rigor:
Describe methods (e.g., software, version) and justify analytical choices.
Address limitations (e.g., sample size, confounding variables).
Validation: Corroborate findings with complementary approaches (e.g., molecular assays, sensitivity analyses).
Reproducibility: Share code/scripts via repositories like GitHub or Zenodo.
Non-Compliance
Submissions lacking adherence to reporting guidelines, ethical standards, or data transparency policies will be returned for revision.
Persistent issues may result in rejection or post-publication corrections.
This policy ensures alignment with global standards for rigorous, ethical, and reproducible health promotion research. For support, contact info@healthpromotionjournal.ir.
- PUBLICATION ETHICS
Health Promotion Scienceadheres to the highest ethical standards in scholarly publishing, guided by the Committee on Publication Ethics (COPE)and its Core Practices. Authors, reviewers, and editors must comply with the following principles to ensure integrity and accountability.
Key Ethical Commitments
- Research Integrity:
- Submissions must be free of research misconduct, including data fabrication/falsification, plagiarism, image manipulation, unethical experimentation, biased reporting, or redundant publication.
- All manuscripts are screened via iThenticate CrossCheckto detect text similarity/plagiarism.
- Human/Animal Rights:
- Studies involving humans or animals require explicit ethical approval and consent (see Section D).
- Transparency:
- Declare conflicts of interest, funding sources, and authorship contributions.
Conflict of Interest (CoI)
- Definition: Any financial, personal, or professional relationship that could influence objectivity (e.g., patents, stock ownership, paid consultancies).
- Requirements:
- Include a CoI Statementin the manuscript, even if none exist (e.g., “The authors declare no conflicts of interest”).
- Disclose allpertinent relationships for every authorduring submission.
- The corresponding author must ensure all co-authors review and approve the final statement.
Funding Disclosure
- Acknowledgments Section: List all funding sources, including grant numbers and institutional support.
- Funder Compliance: Use standardized funder names from the Open Funder Registry.
- Example: “This work was supported by the National Institutes of Health [Grant XYZ123].”
Authorship Criteria
Authors must meet all ICMJE criteria:
- Contribution: Substantial input to study design, data acquisition/analysis, or interpretation.
- Drafting/Revision: Critical intellectual input in writing or revising the manuscript.
- Approval: Final approval of the published version.
- Accountability: Agreement to address accuracy/integrity of the work.
Non-Author Contributions:
- Acknowledge technical support, data curation, or funding assistance in the Acknowledgments(with permission).
CRediT Taxonomy
Health Promotion Science mandates use of the CRediT Taxonomy to specify individual contributions.
Example:
Author A: Conceptualization, Methodology, Writing – Original Draft.
Author B: Formal Analysis, Data Curation, Visualization.
Author C: Supervision, Funding Acquisition, Project Administration.
Roles Include:
- Conceptualization, Methodology, Formal Analysis, Investigation, Writing – Review & Editing, Funding Acquisition.
Author Responsibilities
- Submission: Ensure all authors consent to the submission and authorship order.
- Corrections: Promptly notify the journal of errors post-publication.
- Originality: Confirm the work is unpublished and not under review elsewhere.
Violations & Consequences
- Minor Breaches: Manuscript returned for revision.
- Major Misconduct(e.g., plagiarism, data fraud): Immediate rejection, notification to institutions, and COPE guidance for resolution.
By submitting to Health Promotion Science, authors affirm adherence to these policies. For guidance, consult COPE’s Top 10 Ethics Tips for Authors or contact info@healthpromotionjournal.ir
- AUTHOR LICENSING, OPEN ACCESS, AND PUBLICATION PROCESS
Health Promotion Scienceensures transparent, ethical, and efficient dissemination of research through open access policies and streamlined workflows.
Open Access Licensing
- Creative Commons (CC) Licenses:
- All articles are published under a CC BY-NC 4.0license unless mandated otherwise by funders (e.g., CC BY for UKRI-funded research).
- Author Rights: Retain copyright; public may reuse content for non-commercial purposes with proper attribution.
- Funder Compliance: Verify requirements via Funder Policy Tool.
- Article Processing Charge (APC):
- Levied post-acceptance. Waivers available for low-income regions. View APC Details.
- No Submission Fees: Free to submit and undergo peer review.
- Author Licensing Agreement:
- Corresponding author completes the agreement via Author Services post-acceptance.
Publication Process After Acceptance
- Production Workflow:
- Accepted Article: Transferred to production team; corresponding author notified to sign the license.
- Self-Archiving: Permitted for all versions (submitted, accepted, published) in institutional repositories without embargo.
- Proofs:
- 48-Hour Review: Authors receive HTML proofs via email; must check for errors (e.g., typos, figure alignment).
- Corrections: Limited to technical edits; substantive changes require editorial approval.
- Cover Art Submission (Optional):
- Submit high-resolution images (≥300 dpi) related to the manuscript.
- Cover Image Guidelines include format (TIFF/JPEG), size, and copyright requirements.
Authorship & Contributions
- CRediT Taxonomy:
- Mandatory for specifying roles (e.g., Conceptualization, Formal Analysis).
- Example:
Author A: Methodology, Writing – Original Draft.
Author B: Investigation, Data Curation.
Author C: Supervision, Funding Acquisition.
- Roles: Full CRediT Definitions (e.g., Validation, Visualization, Resources).
- Joint Authorship:
- Joint First/Senior Authors: Add a footnote (e.g., “X and Y contributed equally”).
- Non-Applicable Roles: Omit unused CRediT categories.
Retractions & Confidentiality
- Post-Publication Corrections:
- Follow COPE guidelines for retractions, withdrawals, or expressions of concern.
- Confidentiality:
- All submissions and peer reviews are confidential; authors/reviewers must not disclose details.
Checklist for Authors
Before Submission:
- ☐ ConfirmCRediT rolesand authorship order.
- ☐ Includeethics statements(human/animal studies, clinical trials).
- ☐ Deposit data in aFAIR-aligned repositoryand add a data availability statement.
- ☐ Discloseconflicts of interestand funding sources.
- ☐ Verify compliance with reporting guidelines (e.g., CONSORT, PRISMA).
Post-Acceptance:
- ☐ Sign CC license agreement via Author Services.
- ☐ Review proofs within 48 hours.
- ☐ Consider submitting cover art for promotional opportunities.
- POST-PUBLICATION
Access and Sharing
Upon publication, Health Promotion Science ensures broad dissemination and engagement with your work:
- Author Notification: Corresponding authors receive an email alert with a link to the published article.
- Open Access: Articles are freely available to read, download, and share under the journal’s Creative Commons license(CC BY-NC 4.0 or funder-mandated alternatives).
- Promotion: Authors are encouraged to use [Author Promotion Toolkit](https://authorservices.com/author-resources/Journal-Authors/Promote-your-article.html) to amplify their work via social media, institutional repositories, and academic networks.
Measuring Impact
Health Promotion Science partners with leading platforms to help authors track and enhance the reach of their research:
- Kudos:
- A free service to create plain-language summaries, share via multimedia channels, and monitor engagement.
- Claim your article on Kudosto increase visibility.
- Altmetric:
- Tracks online attention (news mentions, social media, policy citations) via the Altmetric Badgeon each article.
- View real-time metrics on the article’s webpage.
EDITORIAL OFFICE CONTACT DETAILS
For inquiries related to:
- Submission Status: manuscript tracking, revisions, or technical issues.
- Journal Policies: ethics, data sharing, or authorship criteria.
- Complaints: concerns about editorial processes or misconduct.
Contact:
Editorial Office
Email: info@healthpromotionjournal.ir
Complaints Procedure:
- Submit complaints directly to the above email.
- Issues are addressed confidentially and in alignment with COPE guidelines.
Document Transparency
These guidelines were last updated in 2025-03-24.